Abstract
BACKGROUND: Over the past 25 years research has shifted from predominantly focused on men to research that includes both men and women. In addition, myelodysplastic syndromes (MDS) have only been included in the SEER registry since 2001 and are largely understudied. This has resulted in a knowledge gap in the difference in clinical phenotype, genotype, and outcomes between men and women diagnosed with MDS. The aim of this abstract is to identify those gender-based differences.
METHODS: This was a retrospective study using a large MDS database at Moffitt Cancer Center. We compared baseline clinical and molecular characteristics and outcomes based on gender. Chi-square tests were used for comparing categorical variables and t-test for continuous variables. Kaplan-Meier method was used to compare survival.
RESULTS: The Moffitt Cancer Center MDS data base includes 4413 patients among whom 2922 (66%) were men and 1658 (34%) were women.
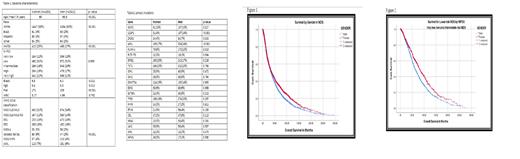
Table-1 summarizes baseline characteristics based on gender. Women were slightly younger (mean age at diagnosis 66.5 versus 69 years for men, p < 0.001). There were more Hispanic/black women than men (9% versus 5%, p =<0.001). Women had slightly lower hemoglobin (mean Hgb 9.4 versus 9.8 g/dl for men, p=0.032) and higher platelet count (mean platelets count 171 versus 136, p < 0.001). More women had del 5/monosomy abnormalities compared to men (p=<0.001), with 353 women (25%) affected. Therapy-related MDS also occurred more often in women (p=<0.001) 413 (25%). More women had isolated del5 q by WHO 2016. (6% vs 2%, p=<0.001). There was no difference in disease risk based on R-IPSS.
There were gender differences in molecular profile. (Table-2). SRSF2 mutation, U2AF1 mutation, ZRSR2 mutation, ASXL1 mutation, and RUNX1 mutations were observed more frequently in men. ZRSR2 was expected.
The median overall Survival (mOS) was longer for women in lower-risk MDS, but not in higher-risk MDS. (Figure- 1 and 2) The overall mOS was 37.5 months (mo) for females compared to 35 mo for males, p=0.002). The mOS for very/low R-IPSS was 81 and 62 mo, for intermediate risk R-IPSS 35 mo vs 33 mo, and for high/very high-risk R-IPSS 16.7 versus 17.4 mo respectively for females and males (p< 0.001). The rate of AML transformation was not different (32% and 34%, respectively for women and men, p =0.16). Women were more likely response to ATG/CSA than men (38% versus 19%, p= 0.04) There were no differences in response to erythroid stimulating agents, hypomethylating agents, lenalidomide treatment or rate of allogeneic hematopoietic stem cell transplant (AHSCT).
CONCLUSION: This retrospective review of a large data base of MDS patients highlights important gender differences in clinical and molecular MDS disease features. We identified differences in rates of selected somatic mutations. Men had more splicing machinery mutations, ASXL-1, and RUNX-1 mutations. Women had better overall survival mainly in lower risk MDS and higher responses to immunosuppressive therapy.
Acknowledgement of Funding: NINR Grant # 1K23NR018488-01A
Tinsley-Vance: Novartis: Consultancy; Celgene/BMS: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Taiho: Consultancy; Fresenius Kabi: Consultancy; Abbvie: Honoraria; Astellas: Speakers Bureau; Jazz: Consultancy, Speakers Bureau. Padron: Kura: Research Funding; Blueprint: Honoraria; Stemline: Honoraria; BMS: Research Funding; Taiho: Honoraria; Incyte: Research Funding. Sweet: Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet: Daiichi Sankyo: Consultancy; Agios: Consultancy; Astellas: Consultancy; AbbVie: Consultancy; ElevateBio Management: Consultancy; Millenium Pharma/Takeda: Consultancy; BerGenBio: Consultancy; Celgene/BMS: Consultancy; Jazz: Consultancy. Kuykendall: BluePrint Medicines: Honoraria, Speakers Bureau; Abbvie: Honoraria; Prelude: Research Funding; PharmaEssentia: Honoraria; Novartis: Honoraria, Speakers Bureau; Incyte: Consultancy; CTI Biopharma: Honoraria; Celgene/BMS: Honoraria, Speakers Bureau; Protagonist: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sallman: Syndax: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau. Komrokji: Geron: Consultancy; Acceleron: Consultancy; AbbVie: Consultancy; BMSCelgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal